Praticiens de la santé
Obtenez de meilleurs résultats thérapeutiques pour vos patients
-
Les tests d'Inagene révèlent quels médicaments et quelles décisions nutritionnelles pourraient contribuer à améliorer les résultats du traitement de vos patients, en fonction de leur profil génétique unique.
-
Des informations génétiques claires et exploitables pour éclairer vos plans de traitement

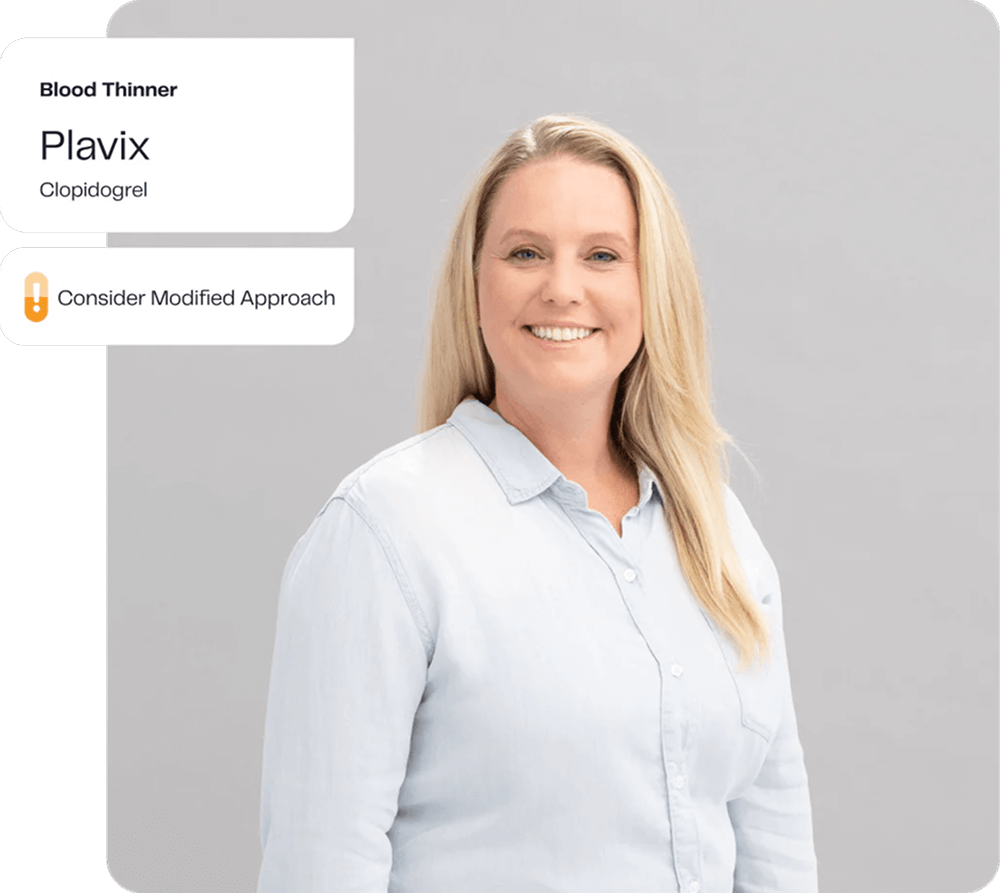
Tests génétiques des médicaments
Les médicaments et les doses qui fonctionnent bien pour un patient ne fonctionnent souvent pas (ou provoquent des effets secondaires inattendus) pour d'autres patients, et cela peut être influencé de manière significative par la génétique. Prendre des médicaments contre la dépression. Seul un tiers environ des personnes souffrant de dépression réagissent bien au premier médicament prescrit ⁵, mais nombre d'entre elles passent des semaines ou des mois à passer d'un médicament à l'autre et d'une dose à l'autre, supportant des symptômes persistants et/ou des effets secondaires.

Tests nutrigénomiques
Des études montrent que les conseils diététiques basés sur l'ADN peuvent accroître la motivation, prévenir les maladies et éviter les intolérances alimentaires. Notre test de nutrigénomique vous permet de travailler avec vos patients pour adapter leur régime alimentaire en fonction de leur ADN. Ce test est fourni en partenariat avec Nutrigenomix, une société de biotechnologie qui se consacre à l'avancement du rôle de la nutrition dans la santé et la performance.
Nos produits
-
Les prestataires de soins de santé partenaires peuvent accéder à tous les tests pour leurs patients à un prix de gros réduit.
Expérience en matière de tests

Le test consiste en un simple prélèvement sur la joue
Étape 1
Votre patient prélève un écouvillon sur sa joue avec notre kit et l'envoie à notre laboratoire dans l'emballage fourni.
Étape 2
Lorsque vos résultats sont prêts, visualisez, téléchargez et partagez vos informations personnalisées.
Étape 3
Votre patient peut discuter des résultats avec vous et d'autres prestataires de soins de santé afin d'optimiser leurs plans de traitement.
-
Vos résultats
Vos résultats personnalisés sont sécurisés, confidentiels et faciles à consulter ou à partager à tout moment et en tout lieu.
-
Réduire les effets secondaires
Minimiser les effets secondaires indésirables et dangereux en comprenant comment vos gènes influencent votre réponse aux médicaments.
-
Éviter les essais et les erreurs
Obtenez des informations personnalisées pour trouver plus rapidement les médicaments les plus susceptibles de vous convenir.
-
Recevoir la dose optimale
Connaître la dose adéquate pour votre corps en fonction de votre constitution génétique unique.
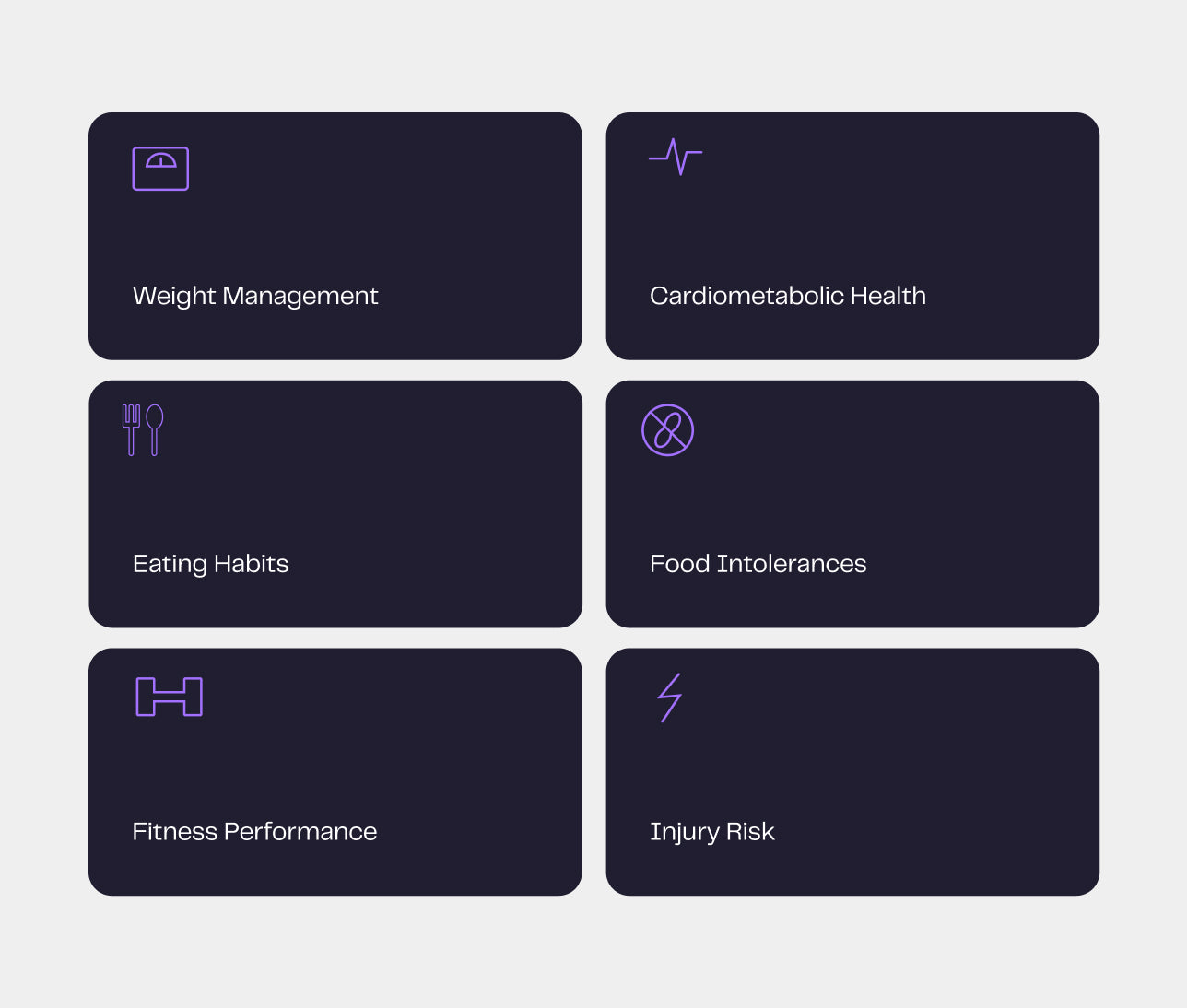
Le rapport Nutrigenomix
Le rapport sur la nutrition et la forme physique de Nutrigenomix fournit des informations qui vous permettent de comprendre la réaction unique de vos patients aux aliments, aux compléments et aux boissons qu'ils consomment, afin qu'ils puissent faire les meilleurs choix en matière d'alimentation. Le rapport Nutrigenomix répartit les résultats et les recommandations en six sections :
- Métabolisme des nutriments
- Intolérances et sensibilités alimentaires
- Santé cardiométabolique
- Gestion du poids et composition corporelle
- Habitudes alimentaires
- Physiologie de l'exercice, condition physique et risque de blessure
La science
Notre laboratoire
Nos tests pharmacogénétiques sont effectués dans notre laboratoire accrédité CLIA, situé à Toronto. La CLIA (Clinical Laboratory Improvement Amendments) garantit que les laboratoires respectent les normes de contrôle de la qualité, que les résultats sont exacts et que les tests effectués sur des spécimens humains fonctionnent comme prévu. Inagene est également titulaire d'une licence d'établissement pour les dispositifs médicaux (n° 9922) délivrée par Santé Canada. Notre technologie de pointe, le système MassARRAY®, combine la spectrométrie de masse, une chimie sensible et robuste et un logiciel d'analyse de données avancé.
Médicaments testés
Inagene propose des tests pharmacogénétiques complets, permettant d'obtenir des informations sur plus de 195 médicaments prescrits pour la douleur, la santé mentale, la cardiologie, le cancer, la santé gastro-intestinale et bien plus encore. Si un médicament ne figure pas sur notre liste, c'est souvent parce qu'il n'y a pas eu suffisamment d'études scientifiques pour établir les facteurs génétiques influençant la réponse d'un individu à ce médicament.
Comment nos recommandations sont-elles formulées ?
Les recommandations contenues dans les informations personnalisées d'Inagene proviennent de consortiums d'experts en pharmacogénétique et d'organismes de réglementation. Ces recommandations fondées sur des données probantes sont ensuite associées à des informations provenant de votre profil ADN unique - fournissant ainsi des conseils personnalisés qui peuvent vous aider à vous sentir mieux, plus rapidement.
En quoi Inagene est-il différent ?
Nous offrons le test le plus complet pour la douleur, la santé mentale, les maladies cardiaques et d'autres conditions, en analysant plus de gènes et de variantes que tout autre test pharmacogénétique au Canada. Contrairement à d'autres tests, les recommandations d'Inagene s'appuient sur des lignes directrices pharmacogénétiques accessibles au public, établies au cours des deux dernières décennies aux États-Unis, en Europe et en Asie par des organismes reconnus et financés par le gouvernement. Nous combinons cette science avec les données du profil d'ADN de vos patients pour fournir des informations personnalisées auxquelles vous pouvez vous fier.


Découvrez l'impact d'Inagene à travers des études de cas réels de patients.
Examiner les résultats d'une étude pilote récente (y compris des cas réels de patients) démontrant comment les résultats des tests d'Inagene peuvent être appliqués pour optimiser les résultats pour les patients souffrant de douleurs chroniques et/ou de troubles de la santé mentale.
-
Comment démarrer
-
Formulaire de référence
Faxez ou envoyez par courrier électronique un formulaire de recommandation contenant les informations relatives au patient. Inagene se charge de contacter le patient, d'organiser le paiement et de veiller à ce que les informations personnalisées soient mises à la disposition du patient et du prestataire de soins de santé.
Consignation
Commandez ou stockez gratuitement des kits de test en consignation et vendez-les directement à vos patients. Le traitement au laboratoire d'Inagene déclenche une facturation d'Inagene à la clinique à un prix de gros.
Codes promotionnels
Recommandez aux patients de commander leur kit sur Inagene.com pour une livraison à domicile en utilisant votre code promo unique.
Commande en temps réel
Faire payer le kit de test aux patients en magasin, puis commander le kit de test gratuitement pour le livrer au domicile du patient. Le traitement au laboratoire d'Inagene déclenche une facturation d'Inagene à la clinique à un prix de gros.
FAQ
Comment ouvrir un compte sur le portail HCP ?
Rendez-vous sur patients.inagene.com, cliquez sur "Register" et suivez les instructions fournies. Veillez à sélectionner "Fournisseur de soins de santé" comme type d'utilisateur. Des instructions détaillées sont également disponibles ici.
Comment la vie privée des patients est-elle assurée ?
Les patients doivent consentir à la réalisation du test génétique, et les prestataires de soins de santé qui commandent/enregistrent le test doivent indiquer qu'ils ont l'autorisation du patient de commander le test et que le patient a eu accès à la politique de confidentialité et de consentement d'Inagene. Les patients peuvent voir qui a accès à leurs résultats via l'onglet "Partage" de leur portail et peuvent supprimer l'accès à tout moment.
Comment les résultats sont-ils partagés entre le pharmacien/le médecin et le patient ?
Le prestataire de soins ou son patient peut enregistrer le kit de test sur le portail Inagene en utilisant le code unique à 14 chiffres qui se trouve dans le kit. À la fin de l'enregistrement, vous pouvez choisir de partager les résultats avec une autre personne en saisissant son adresse électronique (par exemple, le prestataire de soins de santé peut donner un CC à son patient). La personne qui a enregistré le kit et la personne à qui l'on a demandé de partager les résultats recevront toutes deux une notification lorsque les résultats seront disponibles. Les résultats peuvent être partagés ultérieurement en sélectionnant l'onglet "Partage" du portail et en ajoutant un nouvel accès. Les personnes peuvent voir et gérer qui a accès à leurs résultats et supprimer l'accès si nécessaire. Le code à 14 chiffres ne peut être utilisé qu'une seule fois. Le partage peut être géré ou supprimé dans la section "Partage".
Que se passe-t-il si les prestataires de soins de santé ou leurs patients ont des questions auxquelles je ne suis pas en mesure de répondre ?
Les questions fréquemment posées pour aider les patients à consulter sont disponibles sur le portail de l'utilisateur et sont mises à jour régulièrement. La fonction "chat" est également facilement accessible à partir du système de portail et les questions seront transmises si nécessaire au membre de l'équipe d'Inagene le mieux à même de répondre à la question. Les questions peuvent également être envoyées à info@inagene.com.
Mon patient souhaite discuter des tests Inagene avec un autre membre de son équipe soignante avant de les commander. Quelles sont les informations qu'il peut apporter ?
Téléchargez un résumé pour les prescripteurs (avec des liens utiles et les coordonnées d'Inagene) qui peut être remis à votre patient pour qu'il l'envoie ou l'apporte à son prescripteur.
-
Références
-
- La pharmacogénétique à grande échelle : Une analyse de la biobanque britannique
- Haerdtlein A, Debold E, Rottenkolber M, et al. Which Adverse Events and
Which Drugs Are Implicated in Drug-Related Hospital Admissions ? A
Systematic Review and Meta-Analysis. J Clin Med. 2023;12(4):1320.
Publié le 7 février 2023. doi:10.3390/jcm12041320 - Swen JJ, van der Wouden CH, Manson LE, et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions : an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401(10374):347-356. doi:10.1016/S0140-6736(22)01841-4
- Arnone D, Omar O, Arora T, et al. Effectiveness of pharmacogenomic tests including CYP2D6 and CYP2C19 genomic variants for guiding the treatment of depressive disorders : Systematic review and meta-analysis of randomised controlled trials. Neurosci Biobehav Rev. 2023;144:104965. doi:10.1016/j.neubiorev.2022.104965
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term
outcomes in depressed outpatients requiring one or several treatment
steps : a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
doi:10.1176/ajp.2006.163.11.1905